Features
Pulp
Technical paper: White liquor quality during kraft pulping
Exploring how the quality of pulp is dependent on the quality of white liquor used for cooking
May 31, 2022 By Augusto Quinde
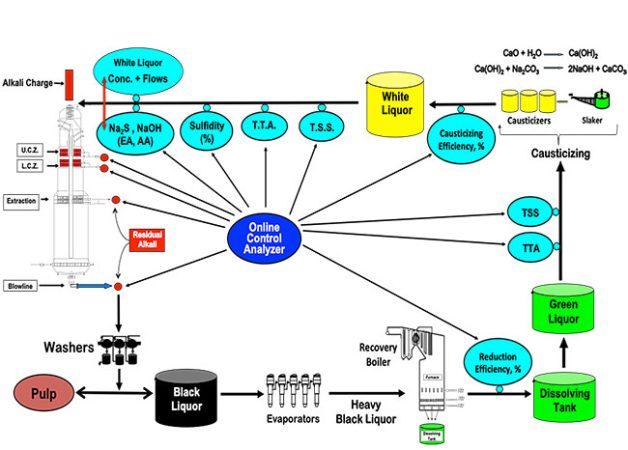
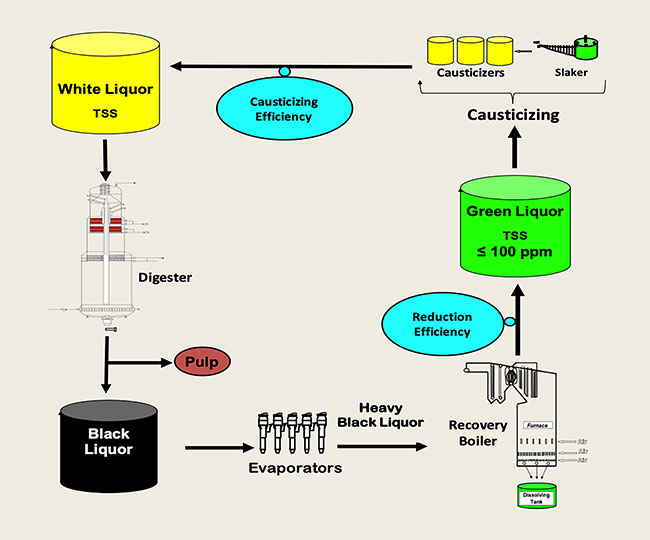 Figure1. Important targets in the causticizing area
Images: Augusto Quinde
Figure1. Important targets in the causticizing area
Images: Augusto Quinde
During continuous cooking operations there is a long list of parameters for monitoring that include three groups. These are wood chip quality, white liquor quality and cooking control.
The main purpose of a causticizing plant is to generate high quality white liquor (Na2S + NaOH) in sufficient quantities to secure steady pulping operations to maintain profitable pulp production operations that comply with environmental and government regulations.
White liquor preparation comprises the following steps:
- Concentrating black liquor;
- Burning concentrated black liquor in a recovery boiler to generate smelt consisting of sodium carbonate (Na2CO3) and sodium sulfide (Na2S);
- Dissolving smelt in weak wash liquors in the dissolving tank to form green liquor that is sent to a green liquor clarifier to remove “dregs” or unburned carbon and other inorganics;
- Slaking calcium oxide (CaO) in the slaker to produce calcium hydroxide [Ca(OH)2 or “slaked lime”] and then completing causticizing reactions in the “causticizers” to convert Na2CO3 to NaOH plus CaCO3 (calcium carbonate or “lime mud”);
- Transferring white liquor to the white liquor clarifier to remove the “lime mud” [CaCO3 and remaining Ca(OH)2];
- Valcining lime mud in a lime kiln to form “reburned lime” or “quick lime” or CaO to be used again in the slaker;
- Sending the clarified white liquor (Na2S + NaOH) to the digester.
Due to environmental regulations, kraft pulp producers are setting trends towards increasing closure of pulp mills by integrating filtrates from the bleach plant and pulp mill into the recovery system. Therefore, this is a big problem that needs to consider not only the inherent chemical components of the dregs from the recovery boiler (such as, unburnt carbon, silica, non-process elements or NPE, etc.) but also the additional chemical waste coming from the bleach plant (Parsad et al. 1996).
A few kraft mills consider causticizing plants as dumping areas and/or unintentionally neglect them off technical assistance or improvement. Fortunately, green liquor clarifiers can be seen as suitable “kidneys” for control and removal of deadload and non-process elements. However, everything has a limit and it is better not to exceed it. The farther apart you are from a paper-machine, the less attention you receive from upper management and the causticizing plant is at the opposite end.
Poor green liquor quality has negative effects on all downstream operations starting with causticizing efficiency and/or white liquor clarification and/or lime mud quality and/or lime kiln operations (Shrinath and Buettner, 2000). Typical dregs content in green liquor is 700-1500 mg/l (Tormala and Markusson, 2013). If the dregs are not properly removed from the green liquor, then the lime mud generated will have more separation problems (such as, settling and filtering) (Azgomi, F. 2014). This paper discusses some aspects of the white liquor quality based on the amount of deadload and its chemical composition.
Variables during causticizing operations
Generating high quality white liquor is a very difficult task due to numerous variables involved during its preparation, starting in the recovery boiler and going through the causticizing operations. Most chemical reactions take place in the recovery boiler, slaker and causticizers and the remaining unit operations involve the separation of liquids and unwanted solids.
Important considerations for optimal separations should be taken in the following stages: green liquor; slaker; causticizing; white liquor clarification; lime kiln, etc. (Sanchez 2007). These variables affect important parameters of the white liquor that is produced through a closed causticizing operation that involves three cycles – calcium cycle; sodium cycle; and sulfur cycle.
Important parameters and targets in the causticizing area
Some important parameters and respective targets change from mill to mill. These are, white liquor concentration in gpl or lb/ft3; sulfidity (percentage); effective alkali (percentage); and total titratable alkali (TTA). However, other parameters must have very specific targets for optimal performance of causticizing operations like: green liquor TSS (total suspended solids ≤ 100 ppm) (Shrinath and Buettner, 2000); white liquor TSS (≤ 20 ppm) (Sanchez 2007); recovery boiler reduction efficiency (90 to 94 percent); and causticizing efficiency (80 to 83 percent). The Na2S passes through the causticizing step unchanged (Tran and Vakkilainnen, 2016).
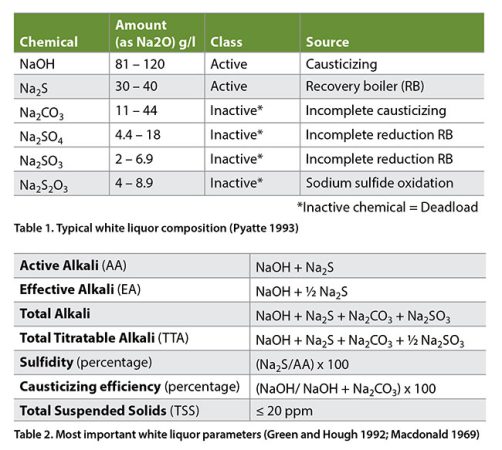
Representative composition of white liquor – The most common compounds present in white liquors are given in Table 1.
Important white liquor parameters related to its composition and properties are given in Table 2.
Deadload origins: How, why and when does it happens?
The white liquor active chemicals (NaOH + Na2S) react during pulping and then are washed out from the pulp into the black liquor, later sent to the evaporators and finally to the recovery boiler. The original chemicals going into the digester are recovered in the 97 to 98 percent range. Most of the sodium sulfate (Na2SO4) is reduced to sodium sulfide (Na2S) in the recovery boiler at reduction efficiencies around 90 to 94 percent and the sodium carbonate (Na2CO3) is converted to NaOH in the causticizers with recausticizing efficiencies in the range 80 to 84 percent (Tran and Vakkilainnen, 2016). See equations 1 and 2.

Equations 1 and 2
Based on the above reduction efficiency and causticizing efficiency, not all of the sodium sulfate is reduced to sodium sulfide nor all the sodium carbonate is causticized to sodium hydroxide. Therefore, some sodium sulfate and sodium carbonate recirculate in the system. Furthermore, other compounds like sodium thiosulfate (Na2S2O3), sodium sulfite (Na2SO3), sodium chloride (NaCl), calcium carbonate (CaCO3) and a set of chemicals known as “non-process elements” together constitute what is known as “deadload”. The main deadload chemical components are sodium sulfate (Na2SO4) and sodium carbonate (Na2CO3) being the sodium carbonate by far the largest amount of deadload.
Effects of deadload and scaling deposits
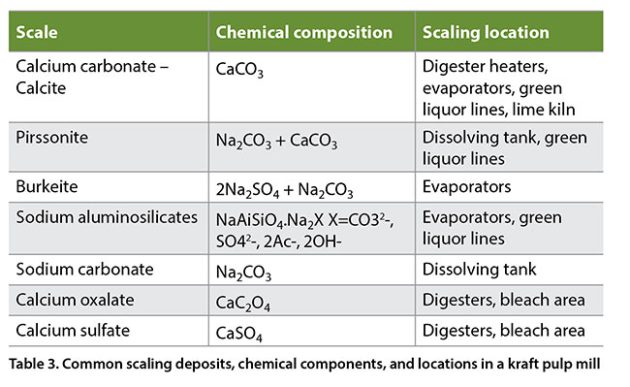
It is evident that high levels of deadload negatively affect the functioning of some equipment, pipelines and pumps with process and economical consequences. The most common effects of deadload in a kraft mill are increased energy usage, greater chemical losses, scaling deposits reducing equipment capacity, downtime for cleaning operations, and lost production, among others.
Scaling takes place almost everywhere in a kraft mill, for example, in digester heaters and evaporators (Duggirala 1994), bleach plants (Shevchenko and Duggirala 2009), dissolving tanks, green and white liquor lines, slaker and causticizers. Removing most scaling deposits is a very expensive cleaning operation. Mills have the following options to consider – hydro-blasting; wood chip leaching treatment at pH 2-3; ion complexing with EDTA or DTPA; acid cleaning; and deactivation of black liquor. The most common scaling deposits in a kraft pulp mill are given in Table 3 (Sithole 2002).
Deadload reduction
Kraft mills can reduce their deadload by reducing sodium carbonate (Na2CO3) by optimizing causticizing efficiencies; reducing sodium sulfate (Na2SO4) by optimizing reduction efficiencies; minimizing chlorides by reducing chloride inputs or purging it; and improving process control measurements at the white liquor plant (Paykkonen 2020). The largest contributor of deadload is sodium carbonate.
Increasing causticizing efficiency reduces more deadload than increasing the reduction efficiency. One percent increase in causticizing efficiency decreases the deadload by six to seven kg/mtp while a one percent increase in reduction efficiency decreases the deadload by two to three kg/mtp (Grace and Tran, 2009). The two most important variables to determine the effect of deadload reduction are the causticizing efficiency (CE percentage) and the reduction efficiency (RE percentage) (Chandra 2004). The primary benefit of reducing the deadload is in energy savings in the digester, evaporators and recovery boiler (Grace and Tran, 2009).
Optimization of causticizing operations
Here are some recommendations to improve causticizing operations and to minimize scaling deposits in the equipment.
- Having a good green liquor and white liquor plant design that must include buffering units to reduce process variations;
- Maintaining the weak wash stream with 100 to 300 ppm suspended solids to help dead load scaling promoters to precipitate in the bulk rather than on equipment walls and/or piping;
- Insulating all green liquor lines properly;
- Properly controlling (automatically) the dissolving tank
- Reducing TTA variations to make correct lime dosage possible (Azgomi, F. 2014, Zuver 2021);
- Monitoring the sources of non-process elements (NPE) into the system to avoid settling issues;
- Purging metals efficiently with dregs removal;
- Switching lines between the raw green liquor (RGL) and weak wash (WW) (Sanchez 2007);
- Minimizing temperature drops across the heat exchanger at the green liquor clarifier to ensure temperatures in the slaker between 100 to 104°C (Green and Hough, 1992);
- Minimizing the deadload by increasing reduction and causticizing efficiencies;
- Searching for appropriate scaling prevention strategies; and
- Optimizing usage of anti-scaling polymers.
Optimization of the causticizing process can be accomplished by using the appropriate online control strategy that should include at least a green liquor TTA-control and a white liquor causticizing efficiency-control (Zuver 2021).
Online control of the kraft recovery cycle
Most kraft mills working at 40 percent or 60 percent over the manufacturer design must properly adjust new pulping parameters that include two very important ones: cooking temperatures and/or cooking chemicals. Of these two options, increasing white liquor requirements seems to be more difficult to satisfy, as we need to consider bigger volumes and high-quality cooking chemicals. The most evident effects on the equipment are the reduction of the retention time of most stages in the causticizing plant due to higher production rates that equates in higher liquor flows, higher amount of deadload due to lower efficiencies and consequently lower white liquor quality.
Leaving all the above parameters to be controlled by the operators is risky and an automatic online control system is necessary (Trung and Allison 2015). Online measurements are available for important determinations in several areas of the recovery and causticizing areas that can guarantee the production of excellent white liquor quality. Online analyzers can determine sodium hydroxide (NaOH), sodium sulfide (Na2S), sodium carbonate (Na2CO3) and report active alkali (AA), effective alkali (EA), total titratable alkali (TTA), causticizing efficiency (CE percentage), and sulfidity (S percentage).
A typical online causticizing application can include five points for sampling – green liquor after dissolving tank; before slaker; top of slaker; causticizing vessels; and white liquor line to digester (Valmet 2015). Laboratory analysis requires manual sampling every four hours while online analysis can give results every six to eight minutes. An ideal online control system might be the one shown in Figure 3.
Final remarks
The quality of pulp depends on the quality of the white liquor used for cooking. This white liquor is as good as the quality of the green liquor from which it is generated (Shrinath and Buettner, 2000).
Modern white liquor plants must generate a high and consistent quality of white liquor in order to produce an optimal pulp quality and to avoid fluctuations/disturbances in the digester. PPC
References
- Azgomi, F. (2014) “Impact of Liming Ratio on Lime Mud Settling and Filterability in the Kraft Recovery Process”. PhD thesis. University of Toronto, Canada. pp 136.
- Chandra, Y., (2004) “Alkaline pulping: Deadload reduction studies in chemical recovery system”. MSc thesis, 53 pp. Georgia Institute of Technology. Georgia. USA.
- Duggirala, P.Y. (1994) “Formation of Calcium Carbonate Scale and Control Strategies in Continuous Digesters”. Nalco Company.
- Grace, T.M. and Tran, H., (2009) “The Effect of dead load chemicals in the kraft pulping and recovery system”. TAPPI Journal, July: 18-24.
- Green, R.P. and Hough, G. (1992) “Chemical Recovery in the Alkaline Pulping Processes”. TAPPI Press, 3rd edition. Atlanta, GA.
- Macdonald, R.G., (1969) Pulp and Paper Manufacture, Vol. I, 2nd edition. “The Pulping of Wood”, Joint Textbook Committee of the Paper Industry. TAPPI. pp 360-361.
- Parsad, B., Kirkman, A., Jameel, H., Gratzl, J., and Magnotta, V. (1996) “Mill Closure with High-Kappa Pulping and Extended Oxygen Delignification”. TAPPI J. 79(9):144-152.
- Päykkönen, J. (2020) “Development of measurements and controls in white liquor plant”. Master Thesis. Lappeenranta University of Technology, School of Energy Systems. Lappeenranta, Finland. pp 67.
- Pyatte, J.A. (1993) “Kraft Pulping – A Compilation of Notes” TAPPI Press. Atlanta, GA. pp 64.
- Sanchez, D.R. (2007) “Recausticizing – Principles and Practice”. Kraft Recovery Short Course January 2007.
- Shevchenko, S.M. and Duggirala, P.Y. (2009) “Deposit Management for the Bleach Plant”. TAPPI Engineering, Pulping & Environmental Conference, October 11-14, Memphis, Tennessee.
- Shrinath, A. and Buettner, C. (2000) “A Review of Issues Related to Green Liquor Quality and Approaches towards Improved Clarification”. ABTCP-TAPPI, Oct.23-26, Sao Paolo, Brasil.
- Sitholé, B. (2002) “Scale deposit problems in pulp and paper mills”. Proc. African Pulp and Paper Week.
- Törmala, J. and Markusson, A. (2013) “Comparison of Alternatives for Green Liquor Handling”. SPCI (The Swedish Association of Pulp & Paper Engineers): Sep.26.2013.
- Tran, H. and E.K. Vakkilainnen (2016) “The Kraft Chemical Recovery Process”. pp 8.
- Trung, T. and Allison, B. (2015) “Advanced Online Process Analyzer for Chemical Recovery and Pulp Mill Control”. O PAPEL: 76(1): 47-56.
- Valmet Adv. Technology (2015) “From smelt to white liquor – the final link”.
- Zuver, D. (2021) “Valmet Causticizing Optimization Case Study at Northeastern US Kraft Paper Mill.”
Augusto Quinde is president of AQuinde Pulping Consulting in Vancouver.
Print this page